ITOCHU Announces Conclusion of a Clinical Research Partnership Agreement with A2 Healthcare and NRG Oncology-Japan Affiliated with US National Cancer Institute
June 1, 2023
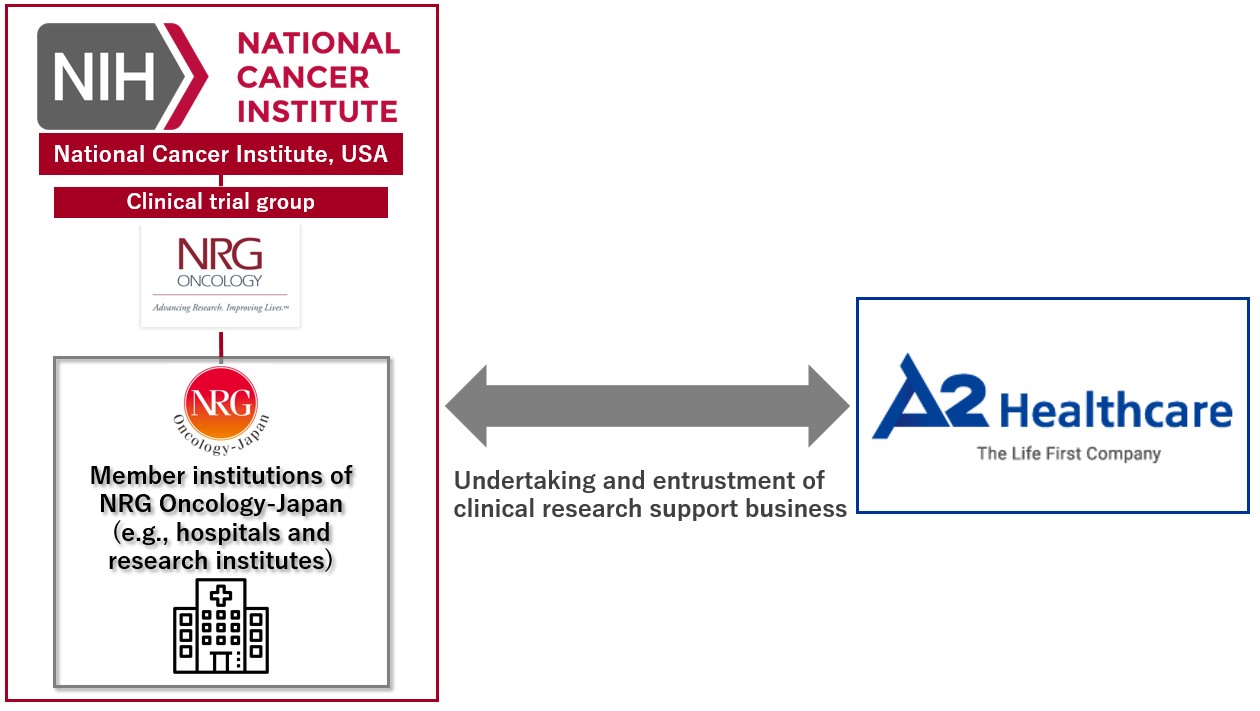
ITOCHU Corporation (headquartered in Minato-ku, Tokyo; Keita Ishii, President & COO; hereinafter “ITOCHU”) announced today that its subsidiary A2 Healthcare Corporation (headquartered in Bunkyo-ku, Tokyo; Hitoshi Kamiya, President & CEO; hereinafter “A2 Healthcare”) concluded the first partnership agreement with NRG Oncology-Japan (hereinafter “NRGJ”) as a CRO*1 in Japan. NRGJ promotes multicenter joint clinical research within Japan as an affiliate of the National Cancer Institute (hereinafter “NCI”). Based on the agreement, A2 Healthcare will continue to engage in the clinical trial support business for drugs that have not yet been approved in Japan to address drug lag and drug loss, which are recent social issues.
Recently, overseas pharmaceutical companies have been reluctant to get involved in the Japanese market. This exacerbates the issues of drug loss, the reduction of the number of new drugs brought into Japan. Of the pharmaceutical products approved in Europe and the USA, 72% of them remain unapproved in Japan as of the end of December 2020.*2 One of the reasons is drug lag, which is the long period of time it takes before a drug that is approved in Europe and the USA is approved in Japan. To address drug loss and drug lag, it is necessary to develop new drugs and apply for approval at the same time in different markets around the world through proactive international joint clinical research in Japan.
NCI is a government agency that has been involved in many anticancer drug development projects in the USA since the 1970s. NCI has led clinical research for many anticancer drugs which have been approved in the USA. NRGJ has supported multicenter trials within Japan for many types of cancer as the lead agency affiliated with NCI for international joint research.
The leading Japanese CRO A2 Healthcare has taken over the execution of business-initiated and doctor-initiated clinical trials for anticancer drug development in Japan, having set up a department dedicated to oncology in 2010. They have carried out over 100 monitoring jobs*3 for a broad range of cancer types, and they have the greatest expertise in supporting cancer drug development among the domestic CROs.
Having concluded the partnership agreement to undertake the clinical research support business for NRGJ's multicenter trials, A2 Healthcare will support the reinforcement and enhancement of NRGJ's investigation system. Led by NCI, this enables more clinical trials to be initiated and more evidence*4 to be produced in Japan for the drugs that are not approved in Japan. It is expected to contribute to increasing the quality of cancer care in Japan and accelerating the delivery of therapies and drugs needed by cancer patients.
ITOCHU transforms business through its “market-in” approach, which is a basic policy included its medium-term management plan, and this effort is in line with this policy. ITOCHU and A2 Healthcare will put their efforts into solving the social issues of drug lag and drug loss and contribute to society by improving healthcare through this initiative focused on the healthcare field.
- *1CRO: An abbreviation for “Contract Research Organization” and an outsourced business engaged in work required in the development of pharmaceutical products (monitoring, data assessment, statistical analysis, etc.)
- *2Source: Annual change in the number of drugs unapproved in Japan and the percentage (tentatively translated, from the website of Pharmaceutical Manufacturers Association)
- *3In monitoring activities, monitors monitor the progress of a clinical trial at centers to guarantee that a clinical trial is accurately performed, recorded and reported in accordance with protocols, standard operating procedures, drug affairs laws and regulations and the Good Clinical Practice (GCP).
- *4Grounds and proof supporting the effectiveness of a drug in treating a disease.
|
|
A2 Healthcare Corporation
| Location | Bunkyo-ku, Tokyo |
|---|---|
| Representative | Hitoshi Kamiya |
| Establishment | July 2003 |
| Business activities | Clinical development, post-marketing surveillance and clinical research services for development of pharmaceutical, medical devices, cellular and tissue-based products and vaccines |
| URL | https://www.a2healthcare.com/en/ |
NRG Oncology-Japan
| Location | Minato-ku, Tokyo |
|---|---|
| Establishment | 2015 |
| Business activities | A global member of NRG Oncology, a multi-institutional clinical research group affiliated with the National Cancer Institute (NCI). NRG Oncology-Japan conducts clinical trials in Japan, focusing on gynecologic cancer, breast cancer and radiotherapy while increasing the types of cancer handled to include cancers in the digestive organs, respiratory organs, urinary organs, head and neck. |